Convert mass to moles for both reactants. (round to 2 significant figures.)
2.50 g CuCl2 equals...

Chemistry, 21.03.2020 22:13 franksjamia
Convert mass to moles for both reactants. (round to 2 significant figures.)
2.50 g CuCl2 equals
| moles
0.50 g Al equals
moles

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
Questions

History, 04.06.2021 01:00



Mathematics, 04.06.2021 01:00





Mathematics, 04.06.2021 01:00

Mathematics, 04.06.2021 01:00


Mathematics, 04.06.2021 01:00


History, 04.06.2021 01:00

Mathematics, 04.06.2021 01:00

Mathematics, 04.06.2021 01:00

Mathematics, 04.06.2021 01:00

History, 04.06.2021 01:00

Mathematics, 04.06.2021 01:00

Mathematics, 04.06.2021 01:00

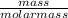
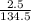 = 0.019moles
= 0.019moles 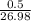 = 0.019moles
= 0.019moles 

