
Chemistry, 21.03.2020 22:58 xxaurorabluexx
The balanced chemical equation for this lab is:
3CuCl2(aq) + 2Al(s) — 3Cu(s) + 2AlCl3(aq)
If 10.5 g copper chloride react with 12.49
aluminum, what is the limiting reactant?
CuCl2
Cu
AICI,

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
The balanced chemical equation for this lab is:
3CuCl2(aq) + 2Al(s) — 3Cu(s) + 2AlCl3(aq)
...
3CuCl2(aq) + 2Al(s) — 3Cu(s) + 2AlCl3(aq)
...
Questions

History, 25.11.2019 12:31



Biology, 25.11.2019 12:31

Mathematics, 25.11.2019 12:31

History, 25.11.2019 12:31


Social Studies, 25.11.2019 12:31

Physics, 25.11.2019 12:31

Mathematics, 25.11.2019 12:31



Mathematics, 25.11.2019 12:31


Health, 25.11.2019 12:31

Computers and Technology, 25.11.2019 12:31

Mathematics, 25.11.2019 12:31

French, 25.11.2019 12:31

History, 25.11.2019 12:31

English, 25.11.2019 12:31

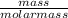
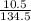 = 0.08moles
= 0.08moles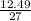 = 0.46moles
= 0.46moles 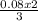 = 0.05moles of Al
= 0.05moles of Al

