
Chemistry, 22.03.2020 05:00 Adolfosbaby
Calculate the maximum numbers of moles and grams of iodic acid (HIO3) that can form when 341 g of iodine trichloride reacts with 151.7 g of water:
ICl3 + H2O → ICl + HIO3 + HCl [unbalanced]

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2


Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

You know the right answer?
Calculate the maximum numbers of moles and grams of iodic acid (HIO3) that can form when 341 g of io...
Questions

Physics, 22.02.2021 22:50


Social Studies, 22.02.2021 22:50




History, 22.02.2021 22:50


Mathematics, 22.02.2021 22:50

Mathematics, 22.02.2021 22:50

Mathematics, 22.02.2021 22:50

Physics, 22.02.2021 22:50






English, 22.02.2021 22:50

History, 22.02.2021 22:50

Mathematics, 22.02.2021 22:50

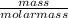
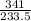 = 1.46moles
= 1.46moles 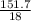 = 8.43moles
= 8.43moles 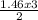 = 2.19 moles
= 2.19 moles 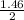 = 0.73moles
= 0.73moles 

