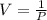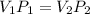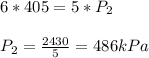Chemistry, 22.03.2020 22:59 LarryJoeseph
3. At a pressure of 405 kPa, the volume of a gas is 6.00 cm
temperature remains constant, at what pressure will the
a gas is 6.00 cm. Assuming the
at what pressure will the new volume be 4.00
cm? Written answer

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
3. At a pressure of 405 kPa, the volume of a gas is 6.00 cm
temperature remains constant, at w...
temperature remains constant, at w...
Questions

Biology, 20.09.2019 09:30

Mathematics, 20.09.2019 09:30

Chemistry, 20.09.2019 09:30






History, 20.09.2019 09:30


Arts, 20.09.2019 09:30

Mathematics, 20.09.2019 09:30


Biology, 20.09.2019 09:30

English, 20.09.2019 09:30

Computers and Technology, 20.09.2019 09:30


English, 20.09.2019 09:30


Mathematics, 20.09.2019 09:30