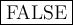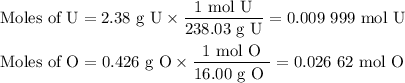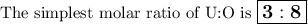Chemistry, 23.03.2020 02:14 selenaK9514
2.38 grams of uranium is heated in a current of air. The resulting oxide
weighs 2.806 grams. When solving for empirical formula, the simplest
molar ratio of uranium is 1.
TRUE or
FALSE

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2


Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
2.38 grams of uranium is heated in a current of air. The resulting oxide
weighs 2.806 grams. W...
weighs 2.806 grams. W...
Questions




Biology, 16.12.2020 18:50


Mathematics, 16.12.2020 18:50



English, 16.12.2020 18:50




Mathematics, 16.12.2020 18:50

Arts, 16.12.2020 18:50

Mathematics, 16.12.2020 18:50

Mathematics, 16.12.2020 18:50


Mathematics, 16.12.2020 18:50

Mathematics, 16.12.2020 18:50