
Chemistry, 23.03.2020 03:32 amandajbrewerdavis
Zinc reacts with iodine in a synthesis reaction. Using a balanced chemical equation for the reaction, determine the percent yield of a 129.0gram sample of zinc was used and 510.6 grams of product is recovered

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

You know the right answer?
Zinc reacts with iodine in a synthesis reaction. Using a balanced chemical equation for the reaction...
Questions




English, 25.12.2019 02:31

Mathematics, 25.12.2019 02:31















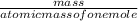
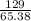
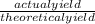 x100 equation 1
x100 equation 1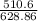 x 100
x 100

