Read the statement.
If 1000 mL of a solution contains 210.0 grams of sodium chloride, wh...

Chemistry, 23.03.2020 04:27 dylancasebere
Read the statement.
If 1000 mL of a solution contains 210.0 grams of sodium chloride, what is the percentage solution (m/v) of this solution?
79%
21%
790%
210%

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Questions

Mathematics, 10.01.2020 16:31

Mathematics, 10.01.2020 16:31

Mathematics, 10.01.2020 16:31

Mathematics, 10.01.2020 16:31

Social Studies, 10.01.2020 16:31




Mathematics, 10.01.2020 16:31


Arts, 10.01.2020 16:31

Mathematics, 10.01.2020 16:31

Mathematics, 10.01.2020 16:31



Mathematics, 10.01.2020 16:31




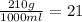 % percentage solution.
% percentage solution.

