Chemistry, 23.03.2020 16:58 mistiehaas
A 850.0 mL cylinder containing B2H6 at a pressure of 0.900 atm is connected by a valve to 1000 mL cylinder containing C6H6 at 608.0 torr pressure. Calculate the partial pressure (torr) of C6H6 when the valve is opened.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
A 850.0 mL cylinder containing B2H6 at a pressure of 0.900 atm is connected by a valve to 1000 mL cy...
Questions

Advanced Placement (AP), 19.05.2021 18:50






Social Studies, 19.05.2021 18:50

Chemistry, 19.05.2021 18:50

Mathematics, 19.05.2021 18:50


Mathematics, 19.05.2021 18:50


Chemistry, 19.05.2021 18:50


Mathematics, 19.05.2021 18:50


Mathematics, 19.05.2021 18:50



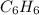 when the valve is opened is 328.6 Torr.
when the valve is opened is 328.6 Torr.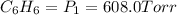

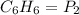
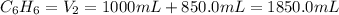
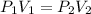 ( Boyle's law)
( Boyle's law)