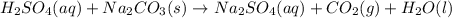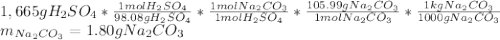Chemistry, 23.03.2020 16:51 tryintopassenioryear
A bottle containing 1,665 g of sulfuric acid (H2SO4, 98.08 g/mol) was spilled in a laboratory. The emergency spill kit contained a full 2.0 kg bottle of sodium carbonate (105.99 g/mol). Is this enough sodium carbonate to neutralize the acid, according to the following reaction
H2SO4(aq) + Na2CO3(s) → Na2SO4(aq) + CO2(g) + H2O(l)
A. Yes, there is more than enough sodium carbonate.
B. Yes, there is exactly enough sodium carbonate-but no excess.
C. No, there is not enough sodium carbonate, but the amount is only about 10% too small.
D. No, there is not nearly enough sodium carbonate.
E. No, the reaction will start going backwards.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1
You know the right answer?
A bottle containing 1,665 g of sulfuric acid (H2SO4, 98.08 g/mol) was spilled in a laboratory. The e...
Questions

Mathematics, 16.07.2019 06:00

History, 16.07.2019 06:00



Chemistry, 16.07.2019 06:00



Mathematics, 16.07.2019 06:00


History, 16.07.2019 06:00

Computers and Technology, 16.07.2019 06:00

Health, 16.07.2019 06:00


Biology, 16.07.2019 06:00

Mathematics, 16.07.2019 06:00



Biology, 16.07.2019 06:00


Mathematics, 16.07.2019 06:00