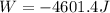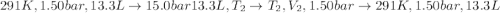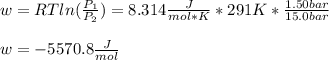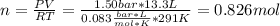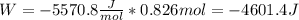An ideal gas described by Ti=291K, Pi=1.50bar, and Vi=13.3L is heated at constant volume until P=15.0bar. It then undergoes a reversible isothermal expansion until P=1.50bar. It is then restored to its original state by the extraction of heat at constant pressure. Calculate w for step 2 (P, Vi, T → Pi, V2, T).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
An ideal gas described by Ti=291K, Pi=1.50bar, and Vi=13.3L is heated at constant volume until P=15....
Questions

Mathematics, 15.01.2020 01:31

Mathematics, 15.01.2020 01:31

Biology, 15.01.2020 01:31


Mathematics, 15.01.2020 01:31

History, 15.01.2020 01:31

Social Studies, 15.01.2020 01:31

Physics, 15.01.2020 01:31



Chemistry, 15.01.2020 01:31

English, 15.01.2020 01:31




Mathematics, 15.01.2020 01:31




Mathematics, 15.01.2020 01:31