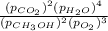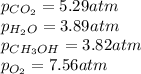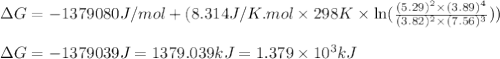A chemist fills a reaction vessel with 3.82 atm methanol (CH, OH) gas, 7.56 am oxygen (O2) gas, 5.29 atm carbon dioxide (CO2) gas, and 3.89 atm water (H0) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: 2CH, OH() + 30266) 2002) + 4H20) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule. x 5 ?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What postulate of the kinetic molecular theory best explains why gases have high fluidity? because collisions between gas particles are elastic, there is no loss of energy as particles flow past each other. because gases consist of large numbers of tiny particles, they spread out and do not come in contact with each other. because the attractive forces between gas particles are negligible, gas particles can glide easily past one another. because the average kinetic energy of gas particles increases as temperature increases, gas particles behave more like a liquid. question 6 compare the compressibility of gases and liquids. support your answer by describing the arrangement of particles in gases and liquids.
Answers: 1


Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
A chemist fills a reaction vessel with 3.82 atm methanol (CH, OH) gas, 7.56 am oxygen (O2) gas, 5.29...
Questions

Health, 23.02.2021 09:00



Mathematics, 23.02.2021 09:00

Arts, 23.02.2021 09:00

Business, 23.02.2021 09:00

Mathematics, 23.02.2021 09:00




Mathematics, 23.02.2021 09:00

Mathematics, 23.02.2021 09:00

English, 23.02.2021 09:00

Mathematics, 23.02.2021 09:00


Social Studies, 23.02.2021 09:00

Mathematics, 23.02.2021 09:00

Mathematics, 23.02.2021 09:00

History, 23.02.2021 09:00

Computers and Technology, 23.02.2021 09:00

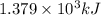
![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f_{(product)}]-\sum [n\times \Delta G^o_f_{(reactant)}]](/tpl/images/0559/0196/f2395.png)
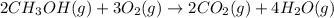
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(CO_2(g))})+(4\times \Delta G^o_f_{(H_2O(g))})]-[(2\times \Delta G^o_f_{(CH_3OH(g))})+(3\times \Delta G^o_f_{(O_2(g))})]](/tpl/images/0559/0196/b933d.png)
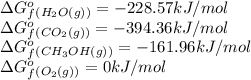
![\Delta G^o_{rxn}=[(2\times (-394.36))+(4\times (-228.57))]-[(2\times (-161.96))+(3\times (0))]\\\\\Delta G^o_{rxn}=-1379.08kJ/mol](/tpl/images/0559/0196/bd425.png)
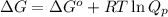
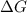 = free energy of the reaction
= free energy of the reaction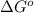 = standard Gibbs free energy = -1379.08 kJ/mol = -1379080 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard Gibbs free energy = -1379.08 kJ/mol = -1379080 J/mol (Conversion factor: 1 kJ = 1000 J)![25^oC=[273+25]K=298K](/tpl/images/0559/0196/0e82f.png)
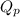 = Ratio of concentration of products and reactants =
= Ratio of concentration of products and reactants =