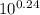Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
A buffer at pH 7.45 is prepared by mixing solutions of KH2PO4 and K2HPO4. Which of the following rat...
Questions

Mathematics, 23.09.2019 03:50



Mathematics, 23.09.2019 03:50

Mathematics, 23.09.2019 03:50


Computers and Technology, 23.09.2019 03:50


Mathematics, 23.09.2019 03:50



Mathematics, 23.09.2019 03:50

Computers and Technology, 23.09.2019 03:50



Mathematics, 23.09.2019 03:50



Health, 23.09.2019 03:50

![\frac{[Base]}{[Acid]}](/tpl/images/0559/1224/ebefd.png) = 1.737
= 1.737