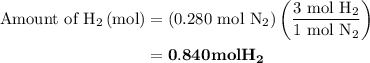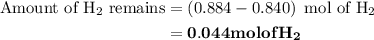Chemistry, 16.01.2020 10:31 balletbella0531
Nitrogen and hydrogen combine at high temperature, in the presence of a catalyst, to produce ammonia. n2(g)+3h2(g)=2nh3(g). assume 0.280 mol of n2 and 0.884 mol of h2 are present initially.. after complete reaction, how many moles of ammonia are . how many moles of h2 . how many moles of n2, what is the limiting or hydrogen)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1



Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Nitrogen and hydrogen combine at high temperature, in the presence of a catalyst, to produce ammonia...
Questions

Spanish, 03.12.2021 04:00

Computers and Technology, 03.12.2021 04:00

Social Studies, 03.12.2021 04:00





Chemistry, 03.12.2021 04:00

Physics, 03.12.2021 04:00

Biology, 03.12.2021 04:00

Computers and Technology, 03.12.2021 04:00

Computers and Technology, 03.12.2021 04:00

Biology, 03.12.2021 04:00


Computers and Technology, 03.12.2021 04:00




Mathematics, 03.12.2021 04:00

Biology, 03.12.2021 04:00

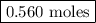
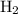 remain on completion of reaction is
remain on completion of reaction is 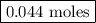
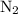 remain on completion of reaction is
remain on completion of reaction is 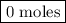
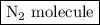
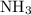 is as follows:
is as follows: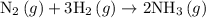
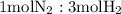
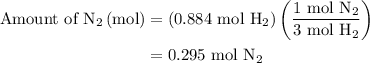
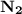 is present in limited quantity and is a limiting reagent.
is present in limited quantity and is a limiting reagent.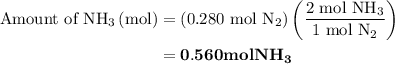
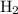 , therefore, the number of moles of
, therefore, the number of moles of