
Chemistry, 23.03.2020 20:21 ranaawilliamsoowl6dk
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemical equilibrium, which of the following explains what happens after the volume of the reaction mixture is increased (assume constant temperature)?
a. The amount of SO3(g) increases and the value for K increases.
b. The amount of SO3(g) decreases and the value for K increases.
c. The amount of SO3(g) stays the same and the value for K decreases.
d. The amount of SO3(g) decreases and the value for K stays the same.
e. The amount of SO3(g) increases and the value for K stays the same

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 12:30
Growing crops in places where major pests don't live using beneficial insects to eat harmful insects using a rat trap instead of a rodenticide developing drought-resistant tomato plants using beneficial insects or natural oils to repel pests planting a different crop every year to fake out the pests keeping food covered to deter ants and rodents developing bean plants with a resistance to fungus picking caterpillars off tomato plants cultivation practice biological control cultural control genetic resistance natural chemicals
Answers: 3
You know the right answer?
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemica...
Questions

History, 08.06.2020 20:57

Mathematics, 08.06.2020 20:57

Mathematics, 08.06.2020 20:57



Mathematics, 08.06.2020 20:57


English, 08.06.2020 20:57

Mathematics, 08.06.2020 20:57

History, 08.06.2020 20:57

Medicine, 08.06.2020 20:57



Mathematics, 08.06.2020 20:57

Mathematics, 08.06.2020 20:57


English, 08.06.2020 20:57



Biology, 08.06.2020 20:57

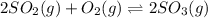
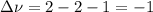
![K=\frac{[SO_3]_{eq}^2}{[SO_2]_{eq}^2[O_2]_{eq}}](/tpl/images/0559/3324/74387.png)


