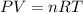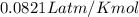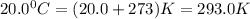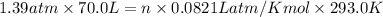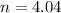Chemistry, 23.03.2020 20:39 negativechill
A 70.0-L steel tank at 20.0°C contains acetylene gas, C2H2, at a pressure of 1.39 atm. Assuming ideal behavior, how many grams of acetylene are in the tank? A 70.0-L steel tank at 20.0°C contains acetylene gas, C2H2, at a pressure of 1.39 atm. Assuming ideal behavior, how many grams of acetylene are in the tank? 4.04 g 105 g 12.9 g 1540 g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

You know the right answer?
A 70.0-L steel tank at 20.0°C contains acetylene gas, C2H2, at a pressure of 1.39 atm. Assuming idea...
Questions


Mathematics, 08.10.2019 19:20



Mathematics, 08.10.2019 19:20

Mathematics, 08.10.2019 19:20



Physics, 08.10.2019 19:20

History, 08.10.2019 19:20

Mathematics, 08.10.2019 19:20

Mathematics, 08.10.2019 19:20





History, 08.10.2019 19:20