
Chemistry, 23.03.2020 20:35 mariahrpoulin9630
Sodium acetate spontaneously crystallizes out of a supersaturated solution upon standing or upon the addition of a seed crystal. Which of the following is true for the thermodynamic quantities of this system for thisprocess?(a)ΔS < 0J/K, ΔH < 0 J(b)ΔS < 0J/K, ΔH > 0 J(c)ΔS > 0J/K, ΔH < 0J(d)ΔS > 0J/K, ΔH > 0J

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Sodium acetate spontaneously crystallizes out of a supersaturated solution upon standing or upon the...
Questions

History, 18.02.2022 06:30




Business, 18.02.2022 06:30

Mathematics, 18.02.2022 06:30

History, 18.02.2022 06:30


Mathematics, 18.02.2022 06:30

Chemistry, 18.02.2022 06:30

Chemistry, 18.02.2022 06:40




Mathematics, 18.02.2022 06:40

Social Studies, 18.02.2022 06:40

Mathematics, 18.02.2022 06:40


Mathematics, 18.02.2022 06:40

Business, 18.02.2022 06:40

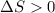 ).
).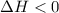 ).
).

