
Chemistry, 23.03.2020 21:11 copelandgarret9972
What concentration of Cl2O remains after a mixture that initially contains [H2O] = 1.00 M and [Cl2O] = 1.00 M comes to equilibrium at 25 °C ? Kc for the reaction is 0.0900.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

You know the right answer?
What concentration of Cl2O remains after a mixture that initially contains [H2O] = 1.00 M and [Cl2O]...
Questions

History, 26.11.2019 20:31


Health, 26.11.2019 20:31


Mathematics, 26.11.2019 20:31

English, 26.11.2019 20:31

Mathematics, 26.11.2019 20:31






Mathematics, 26.11.2019 20:31

Physics, 26.11.2019 20:31

English, 26.11.2019 20:31

History, 26.11.2019 20:31

Mathematics, 26.11.2019 20:31


History, 26.11.2019 20:31

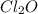 is 0.870 M.
is 0.870 M.![[H_2O]=1.00 M](/tpl/images/0559/4667/b0041.png)
![Cl_2O=[Cl_2O]=1.00 M](/tpl/images/0559/4667/4c14a.png)
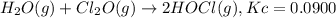
![K_c=\frac{[HOCl]^2}{[H_2O][Cl_2O]}](/tpl/images/0559/4667/da783.png)
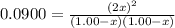
![[Cl_2O]=(1,00-x) M=1.00 M-0.130 M=0.870 M](/tpl/images/0559/4667/c9bd4.png)



