
A 100mL reaction vessel initially contains 2.60x10^-2 moles of NO and 1.30x10^-2 moles of H2. At equilibrium the concentration of NO in the vessel is 0.161M. At equilibrium the vessel contains N2, H2O and H2. What is the value of the equilibrium constant Kc for the following reactions?2H2(g) + 2NO(g) <---> 2 H2O(g) + N2(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
A 100mL reaction vessel initially contains 2.60x10^-2 moles of NO and 1.30x10^-2 moles of H2. At equ...
Questions

History, 19.10.2021 21:40

English, 19.10.2021 21:40







Geography, 19.10.2021 21:40


History, 19.10.2021 21:40

Mathematics, 19.10.2021 21:40

English, 19.10.2021 21:40

Mathematics, 19.10.2021 21:40

History, 19.10.2021 21:40


Mathematics, 19.10.2021 21:40


World Languages, 19.10.2021 21:40

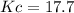
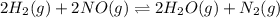
![[H_2]_0=\frac{1.30x10^{-2}mol}{0.1L}=0.130M](/tpl/images/0559/4541/78a1b.png)
![[NO]_0=\frac{2.60x10^{-2}mol}{0.1L}=0.260M](/tpl/images/0559/4541/9b7c0.png)
 , due to the chemical reaction extent, turns out:
, due to the chemical reaction extent, turns out:![x=\frac{[NO]_{0}-[NO]_{eq}}{2} =\frac{0.260M-0.161M}{2}=0.049M](/tpl/images/0559/4541/4c52a.png)
![[H_2]_{eq}=0.130M-2(0.049M)=0.032M](/tpl/images/0559/4541/cb7ca.png)
![[H_2O]_{eq}=2(0.049M)=0.098M](/tpl/images/0559/4541/63a65.png)
![[N_2]_{eq}=0.049M](/tpl/images/0559/4541/f2124.png)
![Kc=\frac{[H_2O]_{eq}^2[N_2]_{eq}}{[H_2]_{eq}^2[NO]_{eq}^2}=\frac{(0.098M)^2(0.049M)}{(0.032M)^2(0.161M)^2} \\\\Kc=17.7](/tpl/images/0559/4541/1d534.png)


