
Chemistry, 23.03.2020 21:45 angelina12386
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The entropy change when 1.85 moles of liquid Co vaporizes at 3097 °C, 1 atm is J/K.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The...
Questions


Spanish, 02.09.2019 14:10

Biology, 02.09.2019 14:10

History, 02.09.2019 14:10


Physics, 02.09.2019 14:10

Social Studies, 02.09.2019 14:10

Mathematics, 02.09.2019 14:10




Mathematics, 02.09.2019 14:10

Biology, 02.09.2019 14:10


Chemistry, 02.09.2019 14:10

English, 02.09.2019 14:10

Geography, 02.09.2019 14:10

Physics, 02.09.2019 14:10

History, 02.09.2019 14:10

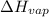 =
= 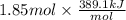
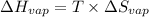
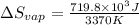
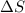 for vaporization is 213.6 J/K.
for vaporization is 213.6 J/K.

