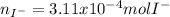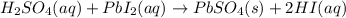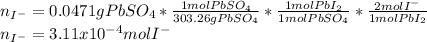Chemistry, 23.03.2020 21:30 Dericktopsom
When H2SO4 is added to PbI2, a precipitate of PbSO4 forms. The PbSO4 is then filtered from the solution, dried, and weighed. If the recovered PbSO4 is found to have a mass of 0.0471 g, how many moles of iodide ions were in the original solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1



Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
When H2SO4 is added to PbI2, a precipitate of PbSO4 forms. The PbSO4 is then filtered from the solut...
Questions

Chemistry, 27.10.2019 05:43

Mathematics, 27.10.2019 05:43

Physics, 27.10.2019 05:43

Spanish, 27.10.2019 05:43


Social Studies, 27.10.2019 05:43

Biology, 27.10.2019 05:43



History, 27.10.2019 05:43


History, 27.10.2019 05:43

Social Studies, 27.10.2019 05:43


Chemistry, 27.10.2019 05:43