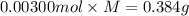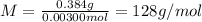A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrated with aqueous NaOH. If 30.0 mL of 0.100 M NaOH is required to reach the equivalence point, what is the molar mass of HA?
(a) 211 g/mol
(b) 128 g/mol
(c) 81.0 g/mol
(d) 37.0 g/mol
(e) 20.3 g/mol

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrat...
Questions





Mathematics, 16.09.2019 19:00

Spanish, 16.09.2019 19:00

English, 16.09.2019 19:00


Social Studies, 16.09.2019 19:00

History, 16.09.2019 19:00

Mathematics, 16.09.2019 19:00

Mathematics, 16.09.2019 19:00

Geography, 16.09.2019 19:00

Mathematics, 16.09.2019 19:00

History, 16.09.2019 19:00

Mathematics, 16.09.2019 19:00


History, 16.09.2019 19:00


Health, 16.09.2019 19:00

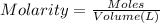
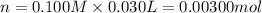
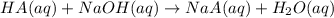
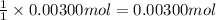 of HA
of HA