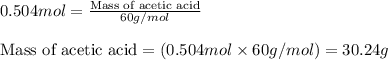Chemistry, 23.03.2020 22:56 j1theking18
In a process for producing acetic acid, oxygen gas is bubbled into acetaldehyde, CH3CHO, containing manganese(II) acetate (catalyst) under pressure at 60°C. 2CH3CHO(l) + O2(g) → 2HC2H3O2(l) In a laboratory test of this reaction, 22.2 g CH3CHO and 12.6 g O2 were put into a reaction vessel. We wish to predict the following: a) How many grams of acetic acid can be produced by this reaction from these amounts of reactants?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
In a process for producing acetic acid, oxygen gas is bubbled into acetaldehyde, CH3CHO, containing...
Questions

English, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30

Biology, 18.05.2021 16:30



Mathematics, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30


History, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30


Mathematics, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30




Spanish, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30

Computers and Technology, 18.05.2021 16:30

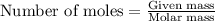 .....(1)
.....(1)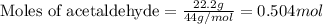
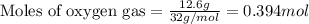
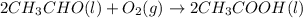
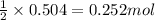 of oxygen gas
of oxygen gas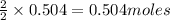 of acetic acid
of acetic acid