
A 18.08-g sample of the ionic compound , where is the anion of a weak acid, was dissolved in enough water to make 116.0 mL of solution and was then titrated with 0.140 M . After 500.0 mL was added, the pH was 4.63. The experimenter found that 1.00 L of 0.140 M was required to reach the stoichiometric point of the titration. a What is the molar mass of ? Molar mass = 129.14 g/mol b Calculate the pH of the solution at the stoichiometric point of the titration.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

You know the right answer?
A 18.08-g sample of the ionic compound , where is the anion of a weak acid, was dissolved in enough...
Questions



History, 20.10.2020 20:01

English, 20.10.2020 20:01




Arts, 20.10.2020 20:01


Mathematics, 20.10.2020 20:01

Advanced Placement (AP), 20.10.2020 20:01




English, 20.10.2020 20:01



Mathematics, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

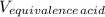 = 1.00 L
= 1.00 L
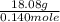
![10^{-4.63]](/tpl/images/0559/8018/9ece0.png)
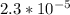
![[A^-]equ = \frac{0.140M*1.00L}{1.00L+0.116L}](/tpl/images/0559/8018/a9cf4.png)
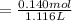
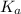 of HA =
of HA = 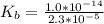
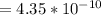
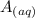 +
+ 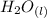
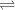
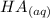 +
+ 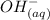
![K_b = \frac{[HA][OH^-]}{[A^-]}](/tpl/images/0559/8018/12da8.png)
![4.35*10^{-10} = \frac{[x][x]}{[0.1255-x]}](/tpl/images/0559/8018/ab7b7.png)
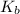 is very small, (o.1255 - x) = 0.1255
is very small, (o.1255 - x) = 0.1255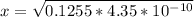
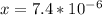
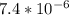 ]
]

