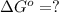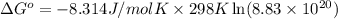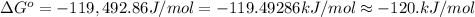In the activity, click on the E∘cell and Keq quantities to observe how they are related. Use this relation to calculate Keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. The two half-reactions that occur in the cell are
Cu2+(aq)+2e−→Cu(s) and Co(s)→Co2+(aq)+2e−
The net reaction is
Cu2+(aq)+Co(s)→Cu(s)+Co2+(aq)
Use the given standard reduction potentials in your calculation as appropriate. ( Keq=5.88*10^20)
In the activity, click on the Keq and ΔG∘ quantities to observe how they are related.
Calculate ΔG∘ using this relationship and the equilibrium constant (Keq) obtained in Part A at T=298K: Keq=5.88*10^20

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
In the activity, click on the E∘cell and Keq quantities to observe how they are related. Use this re...
Questions


Mathematics, 23.03.2021 21:40


Chemistry, 23.03.2021 21:40




Mathematics, 23.03.2021 21:40


Physics, 23.03.2021 21:40


Business, 23.03.2021 21:40


Mathematics, 23.03.2021 21:40

English, 23.03.2021 21:40

Social Studies, 23.03.2021 21:40

Mathematics, 23.03.2021 21:40



English, 23.03.2021 21:50

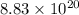 .
.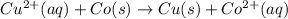
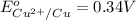
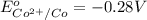
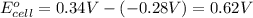
![E^o_{cell}=\frac{0.592}{n}\log[K_c]](/tpl/images/0559/8894/48eca.png)
![0.62 V=\frac{0.0592}{2}\log[K_c]](/tpl/images/0559/8894/f0d8e.png)
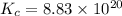
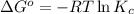
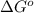 = standard Gibbs free energy =
= standard Gibbs free energy = 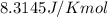
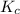 = Equilibrium constant
= Equilibrium constant