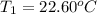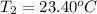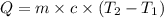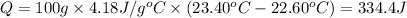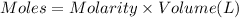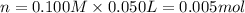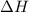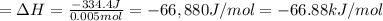Chemistry, 24.03.2020 00:29 PrincesssOfficial
In a coffe cup calorimeter, 50.0mL of 0.100M of AgNO3 and 50mL of 0.100M HCl are mixed to yield the following reaction:
Ag+ (aq) + Cl -==> AgCl(s)
The two solutions were initially at 22.60°C, and the final temperature is 23.40°C. How do I calculate the heat that accompanies this reaction in kJ/mol, assuming that the combined solution has a mass of 100g and a specific heat capacity of 4.18 J/g°C.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
In a coffe cup calorimeter, 50.0mL of 0.100M of AgNO3 and 50mL of 0.100M HCl are mixed to yield the...
Questions


English, 02.07.2019 17:10

Mathematics, 02.07.2019 17:10



Mathematics, 02.07.2019 17:10

Mathematics, 02.07.2019 17:10

History, 02.07.2019 17:10

Mathematics, 02.07.2019 17:10





Biology, 02.07.2019 17:10