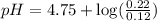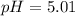Chemistry, 24.03.2020 02:29 doublejojo214
A solution is prepared by mixing 0.12 moles of acetic acid with 0.22 moles of sodium acetate in 1.00 liters of solution. What will be the pH of the solution once equilibrium is established?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1
You know the right answer?
A solution is prepared by mixing 0.12 moles of acetic acid with 0.22 moles of sodium acetate in 1.00...
Questions

Mathematics, 23.10.2020 18:30


Mathematics, 23.10.2020 18:30

English, 23.10.2020 18:30

Biology, 23.10.2020 18:30


Mathematics, 23.10.2020 18:30

Biology, 23.10.2020 18:30

Social Studies, 23.10.2020 18:30

English, 23.10.2020 18:30

Mathematics, 23.10.2020 18:30

History, 23.10.2020 18:30

Mathematics, 23.10.2020 18:30

Mathematics, 23.10.2020 18:30

Health, 23.10.2020 18:30

Chemistry, 23.10.2020 18:30

Mathematics, 23.10.2020 18:30



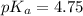
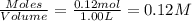
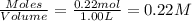
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0560/3046/e961a.png)