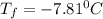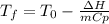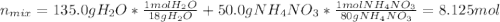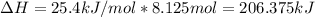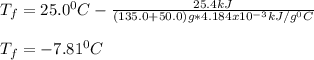Chemistry, 24.03.2020 02:26 ellaemtagedeane
Some instant cold packs contain ammonium nitrate and a separate pouch of water. When the pack is activated by squeezing to break the water pouch, the ammonium nitrate dissolves in water and the pack gets cold. The heat of solution for ammonium nitrate is 25.4 kJ/mol.
a) Is the dissolution of ammonium nitrate endothermic or exothermic?
b) A cold pack contains 135.0 g of water and 50.0 g of ammonium nitrate. What will be the final temperature of the activated cold pack, if the initial temperature is 25.0 degree C? (Assume that the specific heat of the solution is the same as that for water, 4.184 J/g degree C and no heat is lost).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
Some instant cold packs contain ammonium nitrate and a separate pouch of water. When the pack is act...
Questions

Mathematics, 05.05.2020 00:16

Mathematics, 05.05.2020 00:16

Mathematics, 05.05.2020 00:16

Biology, 05.05.2020 00:16

Mathematics, 05.05.2020 00:16

Mathematics, 05.05.2020 00:16




Mathematics, 05.05.2020 00:16

Mathematics, 05.05.2020 00:16




Mathematics, 05.05.2020 00:16


Mathematics, 05.05.2020 00:16

History, 05.05.2020 00:16