
Chemistry, 24.03.2020 02:44 musicaljay1276
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g) Which statement is correct about the complete combustion of 3.00 mole of propane, C3H8? \rm C_3H_8(g) + 5 O_2(g) --> 3 CO_2(g) + 4 H_2O(g)Which statement is correct about the complete combustion of 3.00 mole of propane, \rm C_3H_8?1. 12.00 mol H2O are produced.2. 3.00 g CO2 are produced.3. 3.00 mol CO2 are produced.4. 12.00 g H2O are produced

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g...
Questions



Mathematics, 10.02.2021 09:20


Medicine, 10.02.2021 09:20


English, 10.02.2021 09:20



History, 10.02.2021 09:20

English, 10.02.2021 09:20


Mathematics, 10.02.2021 09:20


Mathematics, 10.02.2021 09:20

Mathematics, 10.02.2021 09:20


Mathematics, 10.02.2021 09:20

English, 10.02.2021 09:20

Mathematics, 10.02.2021 09:20

 ......(1)
......(1)
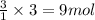 of carbon dioxide
of carbon dioxide
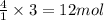 of water
of water


