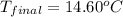Chemistry, 24.03.2020 03:28 bradleylogan78
A student carried heated a 25.00 g piece of aluminum to a temperature of 100°C, and placed it in 100.00 g of water, initially at a temperature of 10.0°C. Determine the final temperature of the system (aluminum and water)
cH2OB4.18J/gc
c Aluminum .900 j/g c

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 09:00
Individuals within populations exhibit some diversity. as a result of possessing slightly different traits, some individuals are better able to survive and reproduce than others. if these individuals changes in the characteristics of the population may occur over time. the cumulative change in these characteristics is known as
Answers: 3

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3
You know the right answer?
A student carried heated a 25.00 g piece of aluminum to a temperature of 100°C, and placed it in 100...
Questions

Biology, 09.12.2020 20:50

History, 09.12.2020 20:50

Social Studies, 09.12.2020 20:50


English, 09.12.2020 20:50



Computers and Technology, 09.12.2020 20:50

Mathematics, 09.12.2020 20:50

Mathematics, 09.12.2020 20:50

Mathematics, 09.12.2020 20:50


Mathematics, 09.12.2020 20:50





Arts, 09.12.2020 20:50


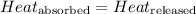
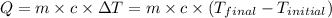
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0560/4279/09236.png) ......(1)
......(1)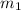 = mass of aluminium = 25.00 g
= mass of aluminium = 25.00 g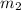 = mass of water = 100 g
= mass of water = 100 g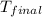 = final temperature = ?°C
= final temperature = ?°C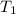 = initial temperature of aluminium = 100°C
= initial temperature of aluminium = 100°C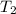 = initial temperature of water = 10°C
= initial temperature of water = 10°C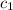 = specific heat of aluminium = 0.900 J/g°C
= specific heat of aluminium = 0.900 J/g°C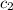 = specific heat of water= 4.18 J/g°C
= specific heat of water= 4.18 J/g°C![25\times 0.900\times (T_{final}-100)=-[100\times 4.18\times (T_{final}-10)]](/tpl/images/0560/4279/4bd6d.png)