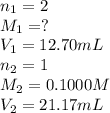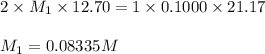Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
A solution of malonic acid, H2C3H2O4 , was standardized by titration with 0.1000 M NaOH solution. If...
Questions

Computers and Technology, 23.11.2019 08:31

Mathematics, 23.11.2019 08:31


Chemistry, 23.11.2019 08:31



Biology, 23.11.2019 08:31






English, 23.11.2019 08:31

Social Studies, 23.11.2019 08:31

English, 23.11.2019 08:31

Mathematics, 23.11.2019 08:31

English, 23.11.2019 08:31

Mathematics, 23.11.2019 08:31


Computers and Technology, 23.11.2019 08:31

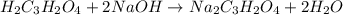
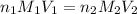
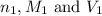 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 
 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.