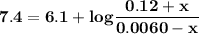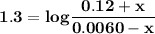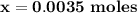Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 Min HCO?3 and 0.0012 M H2CO3 (pKa1 for H2CO3 at body temperature is 6.1).
A. What is the pH of blood plasma?
I got 7.4 for pH which is the correct answer
B. If the volume of blood in a normal adult is 5.0 L, what mass of HCl could be neutralized by the buffering system in blood before the pH fell below 7.0 (which would result in death)?
C. Given the volume from part B, what mass of NaOHcould be neutralized before the pH rose above 7.8?
Express your answer using two significant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3


Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 Min HCO?3 a...
Questions

Mathematics, 30.01.2020 06:00

Mathematics, 30.01.2020 06:00


Mathematics, 30.01.2020 06:00


Biology, 30.01.2020 06:00

Mathematics, 30.01.2020 06:00





Mathematics, 30.01.2020 06:00


History, 30.01.2020 06:01


Mathematics, 30.01.2020 06:01

English, 30.01.2020 06:01

Mathematics, 30.01.2020 06:01


Mathematics, 30.01.2020 06:01

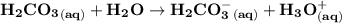
![\mathbf{pH = pKa_1 + log \dfrac{[HCO_3^-]}{[H_2CO_3]}}](/tpl/images/0560/4723/d4625.png)
![\mathbf{pH =6.1 + log \dfrac{[0.024]}{[0.0012]}}](/tpl/images/0560/4723/a7134.png)
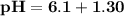
![\mathbf{pH = pKa_1+ log \dfrac{[ salt]}{[acid] }}](/tpl/images/0560/4723/dbb85.png)
![\mathbf{7 =6.1+ log \dfrac{[ 0.024 \times 5 -x]}{[0.0012 \times 5 +x] }}](/tpl/images/0560/4723/43579.png)
![\mathbf{0.9= log \dfrac{[ 0.024 \times 5 -x]}{[0.0012\times 5 +x] }}](/tpl/images/0560/4723/2dcb7.png)
![\mathbf{10^{0.9}= \dfrac{[ 0.024 \times 5 -x]}{[0.0012 \times 5 +x] }}](/tpl/images/0560/4723/8672b.png)
![\mathbf{7.94 = \dfrac{[ 0.024 \times 5 -x]}{[0.0012 \times 5 +x] }}](/tpl/images/0560/4723/b837c.png)
![\mathbf{7.94 = \dfrac{[ 0.12-x]}{[0.006 +x] }}](/tpl/images/0560/4723/81a5e.png)
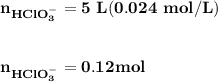
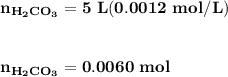
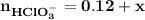
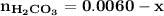
![\mathbf{pH = pKa_1+ log \dfrac{[ HCO_3^-]}{[H_2CO_3] }}](/tpl/images/0560/4723/f8866.png)