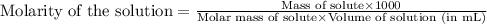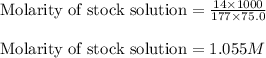The chemical 5-amino-2,3-dihydro-1,4-phthalazine dione, better known as luminol, is used by forensic scientists in analyzing crime scenes for the presence of washed-away blood. Luminol is so sensitive that it can detect blood that has been diluted 10,000 times. A basic solution of luminol is often sprayed onto surfaces that are suspected of containing minute amounts of blood.
Luminol has a molecular weight of 177 g/mol
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 14.0g of luminol into a total volume of 75.0 mL of H2O.
What is the molarity of the stock solution of luminol? I got 1.05M
Before investigating the scene, the technician must dilute the luminol solution to a concentration of 3.00

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

You know the right answer?
The chemical 5-amino-2,3-dihydro-1,4-phthalazine dione, better known as luminol, is used by forensic...
Questions



Physics, 08.09.2019 06:10

Social Studies, 08.09.2019 06:10


Mathematics, 08.09.2019 06:10


Geography, 08.09.2019 06:10


Mathematics, 08.09.2019 06:10

Mathematics, 08.09.2019 06:10




Mathematics, 08.09.2019 06:10