
Consider the reaction 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) When 2 moles of Na react with water at 25°C and 1 atm, the volume of H2 formed is 24.5 L. Calculate the magnitude of work done in joules when 0.55 g of Na reacts with water under the same conditions. (The conversion factor is 1 L · atm = 101.3 J.)

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Consider the reaction 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) When 2 moles of Na react with water at 25...
Questions

Mathematics, 13.02.2021 19:20

Mathematics, 13.02.2021 19:20

Mathematics, 13.02.2021 19:20

Mathematics, 13.02.2021 19:20


Engineering, 13.02.2021 19:20

Mathematics, 13.02.2021 19:20


Social Studies, 13.02.2021 19:20

Mathematics, 13.02.2021 19:20

Mathematics, 13.02.2021 19:20



Mathematics, 13.02.2021 19:20

Chemistry, 13.02.2021 19:20

Mathematics, 13.02.2021 19:20

History, 13.02.2021 19:20


Business, 13.02.2021 19:20

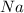
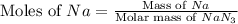
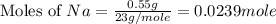
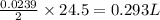 volume of hydrogen gas
volume of hydrogen gas


