
Chemistry, 24.03.2020 05:31 CurlyheadShay
Wrting an equilibrium constant for a reaction sequence Hydrogen is manufactured on an industrial scale by this sequence of reactions: CH,( H, O()Co()+3H2() co(g) +H2O(g) CO2(g) + H2(g) K, The net reaction is: CH4(g)+2H2O(g) CO2(g)+4H2(g) Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K and K If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
You know the right answer?
Wrting an equilibrium constant for a reaction sequence Hydrogen is manufactured on an industrial sca...
Questions

Social Studies, 10.03.2021 18:00

Mathematics, 10.03.2021 18:00

Mathematics, 10.03.2021 18:00

Social Studies, 10.03.2021 18:00






English, 10.03.2021 18:00


Social Studies, 10.03.2021 18:00


History, 10.03.2021 18:00

Arts, 10.03.2021 18:00



Mathematics, 10.03.2021 18:00

Mathematics, 10.03.2021 18:00

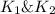 :
: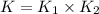
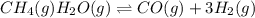
![K_1=\frac{[CO][H_2]^3}{[CH_4][H_2O]}](/tpl/images/0560/6092/ad23e.png)
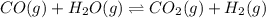
![K_2=\frac{[CO_2][H_2]}{[CO][H_2O]}](/tpl/images/0560/6092/c2b62.png)
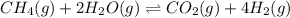
![K=\frac{[CO_2][H_2]^4}{[CH_4][H_2O]^2}](/tpl/images/0560/6092/3e51b.png)
![K=\frac{[CO_2][H_2]^4}{[CH_4][H_2O]^2}\times \frac{[CO]}{[CO]}](/tpl/images/0560/6092/b92b1.png)
![K=\frac{[CO][H_2]^3}{[CH_4][H_2O]}\times \frac{[CO_2][H_2]}{[CO][H_2O]}](/tpl/images/0560/6092/2a778.png)


