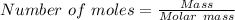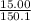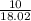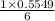Chemistry, 24.03.2020 05:29 lberman2005p77lfi
15.00 g of aluminum sulfide (150.1 g/mol) and 10.00 g of water (18.02 g/mol) react until the limiting reactant is used up. Calculate the mass of H2S (34.08 g/mol) that can be produced from these reactants. Notice that you will need to balance the reaction equation.
___Al2S3(s)+ ___H2O > ___Al(OH)3(s)+ ___H2S(g)
a. 13.89 g
b. 10.21 g
c. 19.67 gd. 9.456 g
e. 1.108 g

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 23.06.2019 12:00
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
You know the right answer?
15.00 g of aluminum sulfide (150.1 g/mol) and 10.00 g of water (18.02 g/mol) react until the limitin...
Questions


Geography, 21.11.2019 11:31

Arts, 21.11.2019 11:31

Mathematics, 21.11.2019 11:31


Mathematics, 21.11.2019 11:31


Mathematics, 21.11.2019 11:31

Mathematics, 21.11.2019 11:31


Mathematics, 21.11.2019 11:31


Mathematics, 21.11.2019 11:31

Chemistry, 21.11.2019 11:31




Computers and Technology, 21.11.2019 11:31

English, 21.11.2019 11:31

English, 21.11.2019 11:31