
Chemistry, 24.03.2020 07:40 lexybellx3
I point
Given the ionization constant equation:
Kw = [H+][OH-]=1.0 x 10-14
For water at 25°C, which statement is true?
1) [H+] = [OH-]
2) [H+]> [OH-]
3) [H+] = 1.0 10-14 M
4) [OH-] = 1.0 × 10-14 M

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
I point
Given the ionization constant equation:
Kw = [H+][OH-]=1.0 x 10-14
For wat...
Given the ionization constant equation:
Kw = [H+][OH-]=1.0 x 10-14
For wat...
Questions

History, 16.02.2021 19:40


Mathematics, 16.02.2021 19:40

Business, 16.02.2021 19:40

Mathematics, 16.02.2021 19:40

Mathematics, 16.02.2021 19:40



Mathematics, 16.02.2021 19:40

History, 16.02.2021 19:40

English, 16.02.2021 19:40


Mathematics, 16.02.2021 19:40

Social Studies, 16.02.2021 19:40

Biology, 16.02.2021 19:40

History, 16.02.2021 19:40

Arts, 16.02.2021 19:40



Mathematics, 16.02.2021 19:40

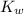 =1.0 ×
=1.0 × 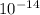 at 25°C
at 25°C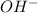 behind) molecule ad gets attached to another water molecule leading to the formation of the hydronium ion(
behind) molecule ad gets attached to another water molecule leading to the formation of the hydronium ion(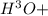 ,hydronium ion).
,hydronium ion).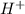 and
and 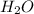 +
+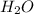 →
→

