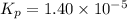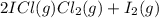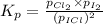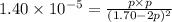Chemistry, 24.03.2020 16:24 faithchambers15
At a temperature below room temperature but with all of the substances still in the gas phase, the equilibrium constant Kp for the decomposition of iodine monochloride (ICl) into I2 and Cl2 is 1.40 × 10–5. If a sealed vessel initially contains 1.70 atm of ICI but no I2 or Cl2. what are the partial pressures of all substances involved in the reaction when it comes to equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
At a temperature below room temperature but with all of the substances still in the gas phase, the e...
Questions



Mathematics, 07.10.2020 06:01


Biology, 07.10.2020 06:01


Mathematics, 07.10.2020 06:01



English, 07.10.2020 06:01



Mathematics, 07.10.2020 06:01

Mathematics, 07.10.2020 06:01

History, 07.10.2020 06:01



English, 07.10.2020 06:01

Mathematics, 07.10.2020 06:01

Mathematics, 07.10.2020 06:01