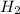Chemistry, 24.03.2020 16:19 juanaaraujo1104
Base your answer on the information below and your knowledge of chemistry. Methanol can be manufactured by a reaction that is reversible. In the reaction, carbon monoxide gas and hydrogen gas react using a catalyst. The equation below represents this system at equilibrium. CO(g) 2H2(g) CH3OH(g) energy. Explain, in terms of collision theory, why increasing the concentration of H2(g) in this system will increase the concentration of CH3OH(g).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1


You know the right answer?
Base your answer on the information below and your knowledge of chemistry. Methanol can be manufactu...
Questions



History, 27.02.2020 23:17

Mathematics, 27.02.2020 23:17




Mathematics, 27.02.2020 23:17


Mathematics, 27.02.2020 23:17


Mathematics, 27.02.2020 23:18

Physics, 27.02.2020 23:18


Computers and Technology, 27.02.2020 23:18





![Rate=k[CO]^x[H_2]^y](/tpl/images/0560/8875/426a0.png)
 and
and