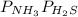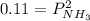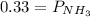Chemistry, 24.03.2020 16:55 ynclankaedon
Ammonium hydrogen sulfide NH4HS(s) decomposes on heating according to the reaction NH4HS(s) ↔ NH3(g) + H2S(g) At 25 °C the equilibrium cnstant of the reaction is Kp = 0.11. What is the total pressure at that temperature in a flask that was initially partially filled with a sizeable amount of NH4HS(s)?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Ammonium hydrogen sulfide NH4HS(s) decomposes on heating according to the reaction NH4HS(s) ↔ NH3(g)...
Questions




Mathematics, 28.04.2021 20:30

Mathematics, 28.04.2021 20:30

Mathematics, 28.04.2021 20:30



Mathematics, 28.04.2021 20:30

Mathematics, 28.04.2021 20:30


Geography, 28.04.2021 20:30

Mathematics, 28.04.2021 20:30



Social Studies, 28.04.2021 20:30

Mathematics, 28.04.2021 20:30

Mathematics, 28.04.2021 20:30

Mathematics, 28.04.2021 20:30