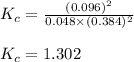Chemistry, 24.03.2020 17:01 emopandabogard8712
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a tank with of sulfur dioxide gas and of oxygen gas, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be . Calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions



Health, 22.02.2021 19:50


Social Studies, 22.02.2021 19:50


World Languages, 22.02.2021 19:50

Physics, 22.02.2021 19:50

Chemistry, 22.02.2021 20:00

Computers and Technology, 22.02.2021 20:00

Mathematics, 22.02.2021 20:00

Mathematics, 22.02.2021 20:00

Mathematics, 22.02.2021 20:00

Mathematics, 22.02.2021 20:00



Computers and Technology, 22.02.2021 20:00

Mathematics, 22.02.2021 20:00

Chemistry, 22.02.2021 20:00

Computers and Technology, 22.02.2021 20:00

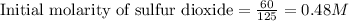
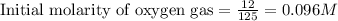
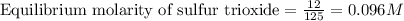
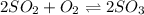
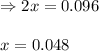
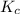 for above equation follows:
for above equation follows:![K_c=\frac{[SO_3]^2}{[O_2]\times [SO_2]^2}](/tpl/images/0560/9411/91096.png)