
Chemistry, 24.03.2020 18:13 doodndns4484
When each of the following equilibria is disturbed by increasing the pressure as a result of decreasing the volume, does the number of moles of reaction products increase, decrease, or remain the same?
1. CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
2. 2CO(g) ⇌ C(s) + CO2(g)
3. N2O4(g) ⇌ 2NO2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
When each of the following equilibria is disturbed by increasing the pressure as a result of decreas...
Questions

Biology, 03.11.2019 18:31


Advanced Placement (AP), 03.11.2019 18:31



Social Studies, 03.11.2019 18:31

History, 03.11.2019 18:31

Advanced Placement (AP), 03.11.2019 18:31

Mathematics, 03.11.2019 18:31

Mathematics, 03.11.2019 18:31


Mathematics, 03.11.2019 18:31

Computers and Technology, 03.11.2019 18:31

Physics, 03.11.2019 18:31


English, 03.11.2019 18:31

English, 03.11.2019 18:31


English, 03.11.2019 18:31

Mathematics, 03.11.2019 18:31

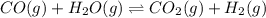 , remain the same.
, remain the same.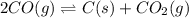 ,increase.
,increase.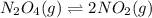 , decrease.
, decrease.

