
Chemistry, 24.03.2020 17:41 adriantheclashroyaln
A 32.5 g iron rod, initially at 22.4 ∘C, is submerged into an unknown mass of water at 63.0 ∘C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 59.7 ∘C. Part A What is the mass of the water? Express your answer to two significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
A 32.5 g iron rod, initially at 22.4 ∘C, is submerged into an unknown mass of water at 63.0 ∘C, in a...
Questions


Mathematics, 12.04.2021 19:10

Mathematics, 12.04.2021 19:10




Mathematics, 12.04.2021 19:10

Social Studies, 12.04.2021 19:10



Chemistry, 12.04.2021 19:10

Mathematics, 12.04.2021 19:10

Mathematics, 12.04.2021 19:10

Mathematics, 12.04.2021 19:10



Mathematics, 12.04.2021 19:10

History, 12.04.2021 19:10

English, 12.04.2021 19:10

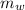 = 39.18 gm
= 39.18 gm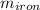 = 32.5 gm
= 32.5 gm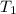 = 22.4°c = 295.4 K
= 22.4°c = 295.4 K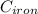 = 0.448
= 0.448 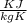

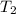 = 336 K
= 336 K  = 59.7°c = 332.7 K
= 59.7°c = 332.7 K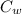 (
(

