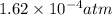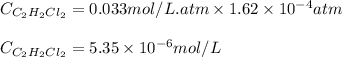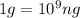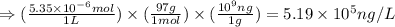Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1
You know the right answer?
A river is contaminated with 0.65 mg/L of dichloroethylene (C2H2Cl2). What is the concentration (in...
Questions



Social Studies, 16.10.2020 09:01


Social Studies, 16.10.2020 09:01

English, 16.10.2020 09:01

English, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01


Physics, 16.10.2020 09:01



Mathematics, 16.10.2020 09:01






Biology, 16.10.2020 09:01

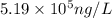
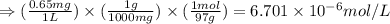
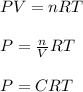
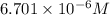
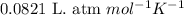
![21^oC=[21+273]K=294K](/tpl/images/0561/0483/32d52.png)
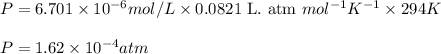
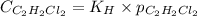
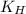 = Henry's constant = 0.033 mol/L.atm
= Henry's constant = 0.033 mol/L.atm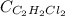 = molar solubility of dichloroethylene gas = ?
= molar solubility of dichloroethylene gas = ?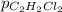 = partial pressure of dichloroethylene gas =
= partial pressure of dichloroethylene gas =