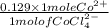Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

You know the right answer?
A bottle in lab is labeled [CoCl2.6H2O] = 0.652 M in 8.433 M HCl. If you determine [CoCl42-] to be 0...
Questions

English, 26.11.2020 23:00


Business, 26.11.2020 23:00







World Languages, 26.11.2020 23:10

English, 26.11.2020 23:10



English, 26.11.2020 23:10


Mathematics, 26.11.2020 23:10

Mathematics, 26.11.2020 23:10



Mathematics, 26.11.2020 23:10

![[Co(H_{2}O)_{6}]^{2} + 4Cl^{-} \rightleftharpoons [CoCl_{4}]^{2-} + 6H_{2}O](/tpl/images/0561/0378/1b7cc.png)
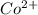 as follows.
as follows.![\frac{0.652 \times 1 mole Co^{2+}}{1 mole [CoCl(H_{2}O)_{6}]^{2+}}](/tpl/images/0561/0378/d9b5b.png)