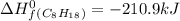For a particular isomer of C8H18, the combustion reaction produces 5113.3 kJ of heat per mole of C8H18(g) consumed, under standard conditions. C8H18(g)+252O2(g)⟶8CO2(g)+9H2O(g)ΔH ∘rxn=−5113.3 kJ/mol What is the standard enthalpy of formation of this isomer of C8H18(g)? ΔH∘f=

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
For a particular isomer of C8H18, the combustion reaction produces 5113.3 kJ of heat per mole of C8H...
Questions

Mathematics, 27.01.2021 17:50


World Languages, 27.01.2021 17:50

Physics, 27.01.2021 17:50

Arts, 27.01.2021 17:50




Mathematics, 27.01.2021 17:50


Medicine, 27.01.2021 17:50

Mathematics, 27.01.2021 17:50

Mathematics, 27.01.2021 17:50

Mathematics, 27.01.2021 17:50

English, 27.01.2021 17:50


Mathematics, 27.01.2021 17:50

Mathematics, 27.01.2021 17:50

Mathematics, 27.01.2021 17:50

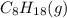 is -210.9 kJ
is -210.9 kJ
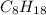 .
.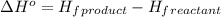
![\Delta H^o=[n_{CO_2}\times \Delta H_f^0_{(CO_2)}+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]-[n_{O_2}\times \Delta H_f^0_{(O_2)+n_{C_8H_{18}}\times \Delta H_f^0_{(C_8H_{18})}]](/tpl/images/0561/1035/28ca9.png)
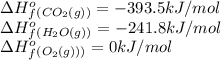
![-511.3kJ/mol=[(8\times -393.5)+(9\times -241.8)]-[(\frac{25}{2}\times 0)+(1\times \Delta H_f^0_{(C_8H_{18})}](/tpl/images/0561/1035/6b6c5.png)