
Chemistry, 24.03.2020 19:17 DaylaReevaFEEVA5040
Tin(IV) sulfide, SnS 2 , a yellow pigment, can be produced using the following reaction. SnBr 4 ( aq ) + 2 Na 2 S ( aq ) ⟶ 4 NaBr ( aq ) + SnS 2 ( s ) Suppose a student adds 47.7 mL of a 0.474 M solution of SnBr 4 to 43.4 mL of a 0.179 M solution of Na 2 S . Identify the limiting reactant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
Tin(IV) sulfide, SnS 2 , a yellow pigment, can be produced using the following reaction. SnBr 4 ( aq...
Questions

Mathematics, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50

Chemistry, 24.02.2021 19:50



Physics, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50

Advanced Placement (AP), 24.02.2021 19:50

English, 24.02.2021 19:50


Mathematics, 24.02.2021 19:50



Mathematics, 24.02.2021 19:50


Biology, 24.02.2021 19:50



Mathematics, 24.02.2021 19:50

Computers and Technology, 24.02.2021 19:50

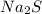 is the limiting reagent
is the limiting reagent  .....(1)
.....(1)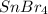 solution = 0.474 M
solution = 0.474 M


 of
of 

