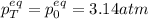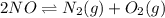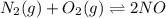Chemistry, 24.03.2020 19:22 brisamauro27
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the total pressure of the mixture at equilibrium is 3.14 atm . N 2 ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO ( g ) K p = 0.101 at 2000 ° C In which direction does the reaction proceed after heating to 2000 °C? The reaction is at equilibrium. The reaction proceeds toward the reactants. The reaction proceeds toward the products.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the...
Questions

Mathematics, 19.01.2021 22:50

English, 19.01.2021 22:50


Mathematics, 19.01.2021 22:50

Mathematics, 19.01.2021 22:50


Mathematics, 19.01.2021 22:50




Mathematics, 19.01.2021 22:50

Mathematics, 19.01.2021 22:50

Mathematics, 19.01.2021 22:50



Mathematics, 19.01.2021 22:50




Chemistry, 19.01.2021 22:50