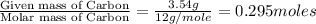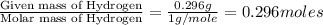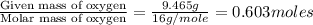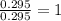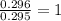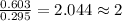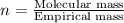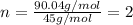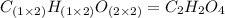Chemistry, 24.03.2020 19:38 cakeisalie6865
A 13.30 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis and 13.00 grams of CO2 and 2.662 grams of H2O are produced. In a separate experiment, the molar mass is found to be 90.04 g/mol. Determine the empirical formula and the molecular formula of the organic compound.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
A 13.30 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis...
Questions

Mathematics, 16.04.2020 21:16



Arts, 16.04.2020 21:16


Mathematics, 16.04.2020 21:16


World Languages, 16.04.2020 21:16

Mathematics, 16.04.2020 21:16






Mathematics, 16.04.2020 21:16





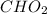 and
and 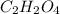
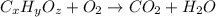
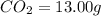
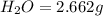
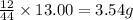 of carbon will be contained.
of carbon will be contained.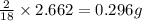 of hydrogen will be contained.
of hydrogen will be contained.