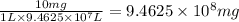Chemistry, 24.03.2020 19:54 charlesmb7985
A water treatment plant applies chlorine for disinfection so that 10 mg/L chlorine is achieved immediately after mixing. The volume of water treated is 25,000,000 gal/day. What is the mass of chlorine needed by this plant per day

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
A water treatment plant applies chlorine for disinfection so that 10 mg/L chlorine is achieved immed...
Questions


Mathematics, 04.02.2020 00:58

History, 04.02.2020 00:58

Mathematics, 04.02.2020 00:58

Chemistry, 04.02.2020 00:58


Biology, 04.02.2020 00:58

Mathematics, 04.02.2020 00:59


Mathematics, 04.02.2020 00:59


Mathematics, 04.02.2020 00:59

History, 04.02.2020 00:59

Mathematics, 04.02.2020 00:59


Geography, 04.02.2020 00:59


Mathematics, 04.02.2020 00:59


Chemistry, 04.02.2020 00:59


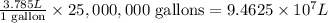
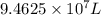 of water, the amount of chlorine applied will be
of water, the amount of chlorine applied will be