

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
2 H2(g) + S2(g) equilibrium reaction arrow 2 H2S(g) At a certain temperature, Kc = 1.30 ✕ 1010 for t...
Questions




Mathematics, 22.07.2019 12:30




History, 22.07.2019 12:30


Health, 22.07.2019 12:30

Business, 22.07.2019 12:30




History, 22.07.2019 12:30

Health, 22.07.2019 12:30


Business, 22.07.2019 12:30

Mathematics, 22.07.2019 12:30

Mathematics, 22.07.2019 12:30

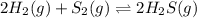
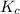 for above equation follows:
for above equation follows:![K_{c}=\frac{[H_2S]^2}{[H_2]^2[S_2]}](/tpl/images/0561/3034/d1522.png)
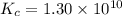
![[H_2]_{eq}=0.00400M](/tpl/images/0561/3034/ad07b.png)
![[S_2]_{eq}=0.00270M](/tpl/images/0561/3034/9642e.png)
![1.30\times 10^{10}=\frac{[H_2S]^2}{(0.00400)^2\times 0.00270}](/tpl/images/0561/3034/e2a23.png)
![[H_2S]_{eq}=23.7,-23.7](/tpl/images/0561/3034/68e27.png)


